We take a lot of things for granted about human biology. Those are the “fundamental truths” we learned all the way in middle school. One such assumption is that a cell division results in the creation of 2 daughter cells. And, it does hold true for most cases. Things are different, however, once we venture into the realm of the pathological, where many generalizations break down.
Out of the many ways mitosis can go wrong is when a defect of the mitotic spindle leads to a tri-, tetra-, pentapolar or higher order division. Toxins, infections and radiation are known causes for this phenomenon. Outside of these influences, multipolar mitoses occur primarily in cancer cells and early in embryonic development.
How multipolar mitosis works
No matter where it happens, if a cell divides into more than 2 new cells, the chromosomes cannot be distributed equally between the daughter cells. The resulting genomic instability favors further mutations, creating a vicious cycle that frequently ends in cancer. Oncogenic viruses such as HPV and HBV express proteins that disrupt the normal functioning of the mitotic spindle. Unsurprisingly, cells in lesions caused by HPV frequently show multipolar mitoses and aneuploidy. That is, an abnormal number of chromosomes.
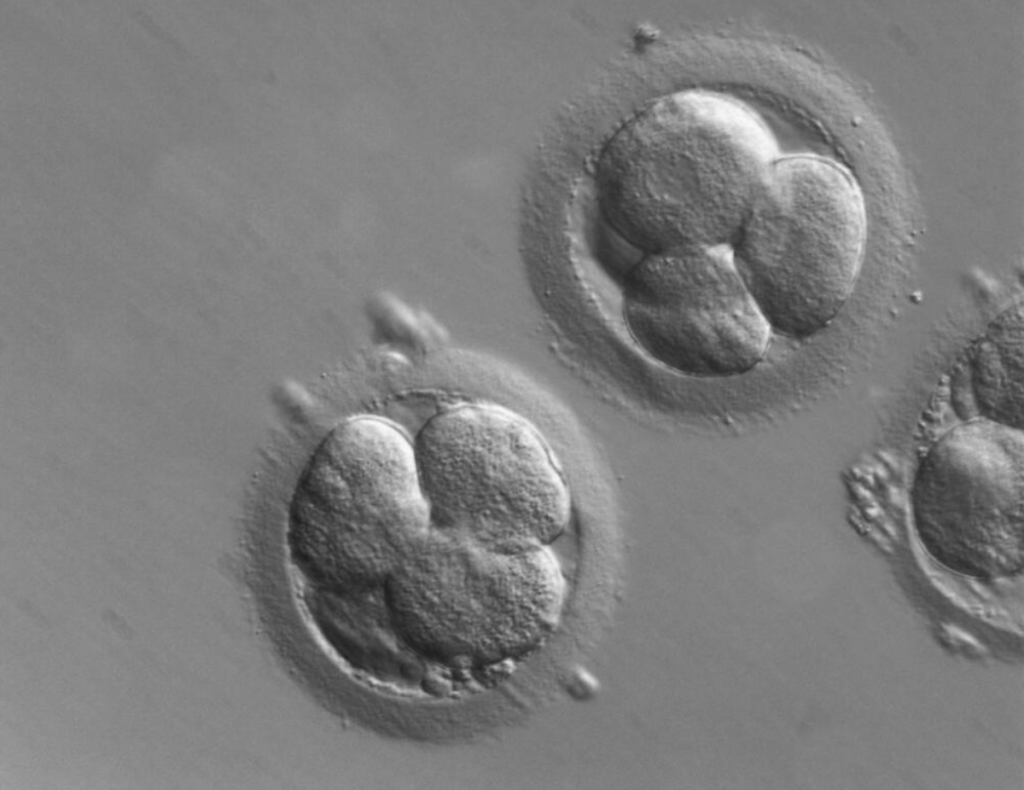
Human zygotes cleave directly into 3 blastomeres (the products of the first embryonic divisions) with a surprisingly high frequency of 12.2% in vitro. Historically, during the early days of in vitro fertilization (IVF), anyone seeing those 3 cells would have assumed that one cell from the first division underwent its second division early. Subsequently, those non-viable embryos would have been implanted. Due to the low percentage of successful pregnancy for one IVF embryo, multiple embryos are implanted per pregnancy. This increases the risk of complications. Human assisted reproduction laboratories around the world are relying more and more on time-lapse monitoring of the zygotes. This allows us to select for the highest quality embryos, on multiple criteria.
The challenges we face
The drawback, however, is that exposure to light negatively affects the number of blastomeres and the ability of the embryo to insert itself into the uterine lining. For reference, the amount of light exposure during regular IVF amounts to 25 minutes, as opposed to ICSI (intracytoplasmic sperm injection), which is 50 minutes.
Aside from this, we use preimplantation genetic diagnosis (PGD) to screen embryos for mutations, but this requires the removal of 1-2 blastomeres. In the case of mosaicism, mutations in the remaining blastomeres remain undetected. So this technique, though useful, has its limitations.
Shortly, the occurrence of multipolar mitoses is particularly noteworthy for its association with genomic instability and cancer development. Furthermore, the high frequency of tripolar divisions in human zygotes highlights the intricacies involved in early embryonic development and assisted reproductive technologies. Advancements such as time-lapse monitoring and preimplantation genetic diagnosis (PGD) have improved embryo selection and screening. However, they come with their own set of challenges. Understanding these phenomena underscores the ongoing need for research on cancer and assisted reproduction.
If you found this article interesting and want to take part in shaping the future, stay tuned and stay curious with us! You can read the main article here; additional references here and here. See you next time.
About the author…
Hello 🙂 My name is Matei and I am a second year medical student who loves science, video games and skiing.
